Lithium-metal batteries could double the range of an electric vehicle. Still, their life cycle is short because they degrade very fast. Stanford scientists found a simple way to extend their lives and improve performance, making the new battery cells ideal for electric vehicles.
Today, Li-ion batteries power almost everything, from radios and mobile phones to headphones, drones, vacuum cleaners, and electric vehicles. Li-ion batteries also serve as energy storage devices for homes or businesses. Because there is no perfect lithium-ion battery, people use different chemistries and packages depending on their use cases.
Most battery cells in modern electric vehicles use an NCA (Nickel-Cobalt-Aluminum) or NMC (Nickel-Manganese-Cobalt) chemistry because they provide high density and long service life. Lithium-ion-phosphate (LFP) cells can offer a price-conscious alternative for budget EVs, trading off energy density for a longer life cycle. However, better lithium-based battery cells could replace current cell types in future electric vehicles.
One of the most promising types is lithium-metal, thanks to a much higher energy density than the current best Li-ion cells. In a lithium-metal battery, the graphite anode typical of current Li-ion batteries is replaced with electroplated lithium metal. This enables it to store twice the energy for the same volume. The lithium metal anode is also lighter than the graphite one, making it ideal for electric vehicles and aircraft. Researchers think lithium-metal batteries could unlock 500-700 miles ranges in the future electric cars.
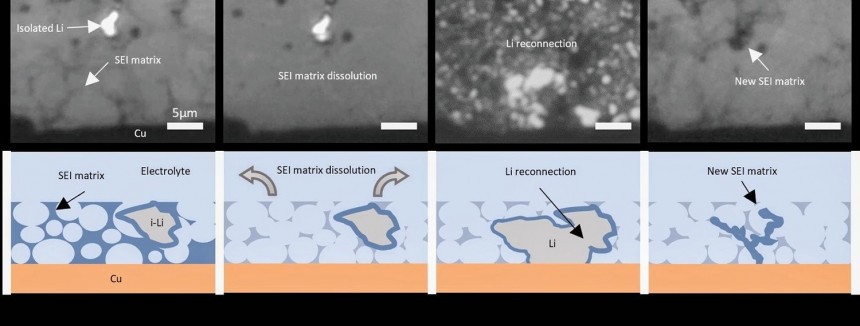
However, the technology has a significant drawback, as lithium-metal batteries degrade relatively fast with charging and discharging. During discharging, micron-sized bits of lithium metal become isolated and get trapped in the solid electrolyte interphase (SEI), a spongy matrix that forms where the anode and electrolyte meet. With every charge and discharge cycle, additional dead lithium builds up, causing the battery to rapidly lose capacity. This is one of the reasons why these battery cells have not yet entered production.
Scientists have been struggling for years to find a solution to this problem by testing various materials and techniques. However, the solution might be more straightforward and cost-effective: simply drain the battery and let it rest for several hours. This is enough for the lithium metal in the SEI matrix to dissolve. During charging, the dead lithium will reconnect with the anode, enabling the battery to store more energy and extend its life cycle.
Letting the battery rest empty for several hours might not be ideal for EV owners. Many still refuse to buy an EV because charging it takes too long compared to filling up a gas tank. However, considering many cars sit idle in a garage or parking lot most of the time, it might not be such a big inconvenience. To eliminate it completely, Stanford scientists found a clever trick.
Most battery packs are made of many modules, so it would be feasible to use them selectively. This way, some modules can regenerate while others power the electric vehicle. It would not be hard to program the battery management system to charge and drain the battery modules as required to keep them in perfect shape. The operation would be transparent to the car's owner, with software taking care of everything.
Most battery cells in modern electric vehicles use an NCA (Nickel-Cobalt-Aluminum) or NMC (Nickel-Manganese-Cobalt) chemistry because they provide high density and long service life. Lithium-ion-phosphate (LFP) cells can offer a price-conscious alternative for budget EVs, trading off energy density for a longer life cycle. However, better lithium-based battery cells could replace current cell types in future electric vehicles.
One of the most promising types is lithium-metal, thanks to a much higher energy density than the current best Li-ion cells. In a lithium-metal battery, the graphite anode typical of current Li-ion batteries is replaced with electroplated lithium metal. This enables it to store twice the energy for the same volume. The lithium metal anode is also lighter than the graphite one, making it ideal for electric vehicles and aircraft. Researchers think lithium-metal batteries could unlock 500-700 miles ranges in the future electric cars.
Scientists have been struggling for years to find a solution to this problem by testing various materials and techniques. However, the solution might be more straightforward and cost-effective: simply drain the battery and let it rest for several hours. This is enough for the lithium metal in the SEI matrix to dissolve. During charging, the dead lithium will reconnect with the anode, enabling the battery to store more energy and extend its life cycle.
Letting the battery rest empty for several hours might not be ideal for EV owners. Many still refuse to buy an EV because charging it takes too long compared to filling up a gas tank. However, considering many cars sit idle in a garage or parking lot most of the time, it might not be such a big inconvenience. To eliminate it completely, Stanford scientists found a clever trick.
Most battery packs are made of many modules, so it would be feasible to use them selectively. This way, some modules can regenerate while others power the electric vehicle. It would not be hard to program the battery management system to charge and drain the battery modules as required to keep them in perfect shape. The operation would be transparent to the car's owner, with software taking care of everything.