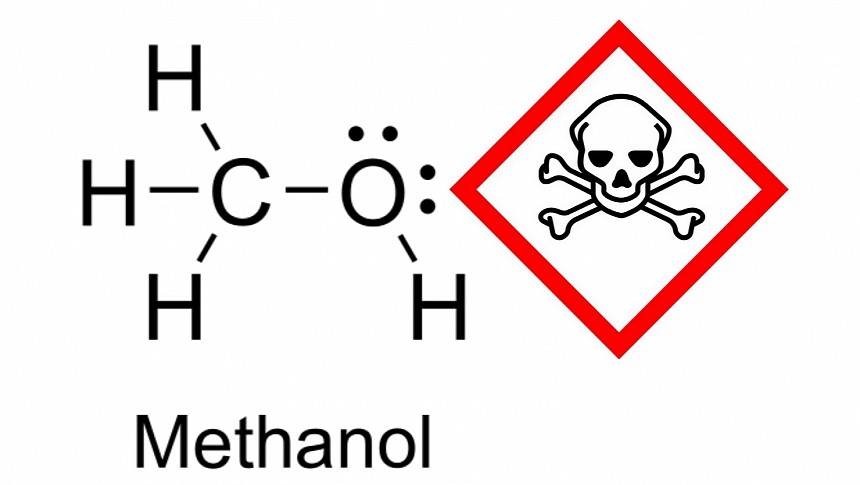
When we discuss electric cars, there's a clear preference for vehicles that work at 800V because they can be lighter and charge faster. The issue is that the higher voltage can be a safety issue for human beings. As a matter of fact, even 400V systems are dangerous, which is why Toroidion only works with 48V. Renewable fuels could be a safer bet if it were not for the methanol preference that most companies promoting them reveal. Well, this alcohol is highly toxic, and you will not see many people mentioning that. They should.
Methanol is the preferred synthetic fuel because it presents a more straightforward chemical structure, with only one carbon atom per molecule. That makes it easier to produce than ethanol, which has two carbon atoms per molecule. On the other hand, it also offers less energy. While ethanol has 70% of the energy content of the equivalent volume of gasoline, methanol presents only 60%.
When methanol enters the body (by ingestion, inhalation, or absorption by the skin), it is metabolized to formic acid. Gasoline is also toxic, but it is not soluble in water, something that methanol is. The alcohol is also less volatile than gasoline, which makes its vapors less detectable. This all makes methanol a more insidious fuel. When poisoning symptoms emerge, it's already too late to treat them. Lethal ingestion can be as low as 15 ml (0.9 cubic inches), but people can go blind or have kidney failure even if they manage to survive. It is definitely not a substance you'd like to handle on a daily basis.
Ask race teams that used methanol in the past or even Brazilian drivers who had to cope with the ethanol shortage that happened by the end of the 1980s. The Brazilian government was forced to import methanol to cope with the crisis, which led to several safety campaigns about how to deal with that fuel. As one of the people who witnessed that shortage, it surprised me to see how many companies are advocating for methanol as a renewable fuel.
In a way, it is understandable that those trying to find alternatives to fossil fuels will pursue synthetic fuels that demand less energy to be manufactured. After all, this renewable solution has to be economically competitive. However, removing fossil fuels from the equation by offering a toxic one as the alternative looks like a poor strategy, especially if the new fuel is to be used in internal combustion engines (ICEs). If vehicles burn it, they will require massive amounts of it.
Let's suppose methanol's main target is to serve solely as a liquid hydrogen reserve, which would make it easier to store and use in fuel cells. Gumpert Automobile is trying to do that. In the video below, the company made a MAN TGE with an electric powertrain run 1,002 kilometers (622.6 miles). It was loaded with sandbags to its full 3,500-kilograms (7,716 pounds) gross vehicle weight (GVW) without stopping a single time for refueling. Sadly, the company did not disclose how big the methanol tank was, but it allowed us to discover that. According to Gumpert, the e-TGE had a fuel consumption of 14.5 liters of methanol per 100 km. That means that the vehicle spent 145 liters of methanol in the 1,002-km trip. If the tank is bigger than that, the special TGE may drive even further.
Gumpert Automobile is pretty happy because methanol has a lower energy content than diesel. When that difference is corrected, it is as if the e-Transporter carried 3.5 metric tons for 1,000 km, spending 6.6 liters of diesel per 100 km. The TGE powered by diesel engines delivers around 9 l/100 km, so Gumpert's solution saves fuel because it needs less energy for the task. That is no surprise, considering the electric powertrain. However, what matters in this discussion is how drivers and workers protect themselves while refueling any vehicle with methanol. As I already mentioned, breathing its gases can also be harmful.
This is something e-fuel defenders will have to figure out before reaching production scale for their solutions. Will it be worth submitting methanol to more industrial steps so that vehicles use ethanol or synthetic gasoline instead? If we are talking about something for ICEs to burn – old or new ones – the answer is definitely yes. If the idea is to have a liquid storage for hydrogen, we'll probably have to discuss it a bit further.
If methanol is easier to produce, it is probably also more straightforward to break down and release hydrogen. A fuel cell electric vehicle (FCEV) that aims to be energy efficient will undoubtedly prefer that, especially if it has a reformer to turn methanol into hydrogen onboard. A way to prevent that is to follow what Toyota is testing in Brazil. The Japanese carmaker put a reformer in an experimental fuel station, which means FCEV drivers will never have to deal with methanol.
This approach will also facilitate hydrogen transportation. Instead of high-pressure pipelines or trucks with special tanks, we could use the same vehicles that currently transport fuel and other liquids. The downside is that the hydrogen vehicles would need high-pressure tanks. A reformer in each car to convert methanol into hydrogen is probably a better call, as the Mercedes-Benz NECAR 3 tried to demonstrate in 1997. Yet, it leads us back to the same dilemma we had before: is it worth the risk of using a toxic alcohol as a hydrogen vehicle? Isn't it worth researching something safe for humans and the environment that plays the same role?
Our rush to find quick solutions for complex problems may lead us to follow equivocal paths. Getting rid of fossil fuels by creating a synthetic and economically feasible alternative is more than desirable, but this option also has to be safe. If it is not, we will be only trading a problem for another one, which may be more complex to solve. More than that, anything that represents risks to human lives should be excluded right off the bat. Why would methanol get a free pass if high voltage is not desirable?
As for the companies promoting methanol, there are only two explanations for them not to mention it is toxic: either they don't know that, or they don't want the public to learn this. If drivers only discover that when the distribution is already solved and methanol-powered vehicles are for sale, they may reject these products, but it will take a while for them to kick the bucket. If they learn about it now, they may help avoid a solution that creates another problem. If we are to use renewable fuels, methanol should not be on the table.
When methanol enters the body (by ingestion, inhalation, or absorption by the skin), it is metabolized to formic acid. Gasoline is also toxic, but it is not soluble in water, something that methanol is. The alcohol is also less volatile than gasoline, which makes its vapors less detectable. This all makes methanol a more insidious fuel. When poisoning symptoms emerge, it's already too late to treat them. Lethal ingestion can be as low as 15 ml (0.9 cubic inches), but people can go blind or have kidney failure even if they manage to survive. It is definitely not a substance you'd like to handle on a daily basis.
Ask race teams that used methanol in the past or even Brazilian drivers who had to cope with the ethanol shortage that happened by the end of the 1980s. The Brazilian government was forced to import methanol to cope with the crisis, which led to several safety campaigns about how to deal with that fuel. As one of the people who witnessed that shortage, it surprised me to see how many companies are advocating for methanol as a renewable fuel.
Let's suppose methanol's main target is to serve solely as a liquid hydrogen reserve, which would make it easier to store and use in fuel cells. Gumpert Automobile is trying to do that. In the video below, the company made a MAN TGE with an electric powertrain run 1,002 kilometers (622.6 miles). It was loaded with sandbags to its full 3,500-kilograms (7,716 pounds) gross vehicle weight (GVW) without stopping a single time for refueling. Sadly, the company did not disclose how big the methanol tank was, but it allowed us to discover that. According to Gumpert, the e-TGE had a fuel consumption of 14.5 liters of methanol per 100 km. That means that the vehicle spent 145 liters of methanol in the 1,002-km trip. If the tank is bigger than that, the special TGE may drive even further.
Gumpert Automobile is pretty happy because methanol has a lower energy content than diesel. When that difference is corrected, it is as if the e-Transporter carried 3.5 metric tons for 1,000 km, spending 6.6 liters of diesel per 100 km. The TGE powered by diesel engines delivers around 9 l/100 km, so Gumpert's solution saves fuel because it needs less energy for the task. That is no surprise, considering the electric powertrain. However, what matters in this discussion is how drivers and workers protect themselves while refueling any vehicle with methanol. As I already mentioned, breathing its gases can also be harmful.
If methanol is easier to produce, it is probably also more straightforward to break down and release hydrogen. A fuel cell electric vehicle (FCEV) that aims to be energy efficient will undoubtedly prefer that, especially if it has a reformer to turn methanol into hydrogen onboard. A way to prevent that is to follow what Toyota is testing in Brazil. The Japanese carmaker put a reformer in an experimental fuel station, which means FCEV drivers will never have to deal with methanol.
This approach will also facilitate hydrogen transportation. Instead of high-pressure pipelines or trucks with special tanks, we could use the same vehicles that currently transport fuel and other liquids. The downside is that the hydrogen vehicles would need high-pressure tanks. A reformer in each car to convert methanol into hydrogen is probably a better call, as the Mercedes-Benz NECAR 3 tried to demonstrate in 1997. Yet, it leads us back to the same dilemma we had before: is it worth the risk of using a toxic alcohol as a hydrogen vehicle? Isn't it worth researching something safe for humans and the environment that plays the same role?
As for the companies promoting methanol, there are only two explanations for them not to mention it is toxic: either they don't know that, or they don't want the public to learn this. If drivers only discover that when the distribution is already solved and methanol-powered vehicles are for sale, they may reject these products, but it will take a while for them to kick the bucket. If they learn about it now, they may help avoid a solution that creates another problem. If we are to use renewable fuels, methanol should not be on the table.