It can recycle waste into valuable graphene; it can regenerate graphite anodes in lithium-ion batteries; it can make low-cost hydrogen from plastic. It's the Flash Joule Heating process developed in the early 2020s by chemist James Tour of Rice University.
Interestingly, one of this university's alums, Louis Brus, is among the Nobel Prize in Chemistry 2023 winners. I wouldn't be surprised if the "Flash Graphene" will get James Tour a Nobel Prize in a few years. It sounds almost miraculous.
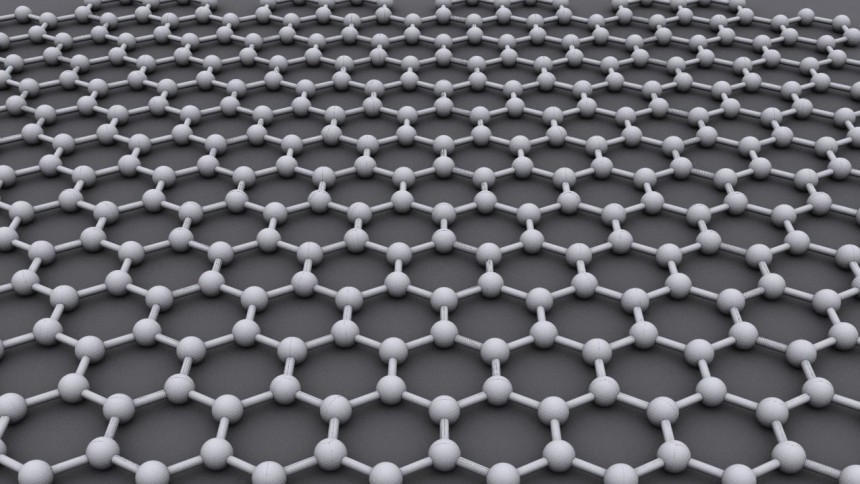
The graphene is like a superhero material in the nanotechnology realm. Ok, Antman, please don't make a scene; we're not getting into Quantum Realm. Quantum mechanics describes aspects of nature at subatomic scales, while nanomaterials are on a near-atomic scale.
Graphene was discovered and scientifically proved in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester. They were later awarded the Nobel Prize in Physics 2010 for "groundbreaking experiments regarding the two-dimensional material graphene."
Wait, a 2-D material in a 3-D world? That's right, graphene is a one-atom-thick nanomaterial with exceptional tensile strength and electrical conductivity – just a few of its fascinating qualities suitable for electronics, energy, composites, or biomedicine.
It's too complicated to explain what graphene really is, so I'll stick to probably the most comprehensive feature: this material is about 100 times stronger than the strongest steel with the same thickness. Simply put, it's better than Spiderman's web.
The graphene market is following the S-curve rule: while in 2012, its global market was valued at under $10 million / €8 million, ten years later, it jumped to around $700 million / €600 million, and the most recent forecasts estimate it will get to about $2.5 billion by 2028.
But get this: these are very conservative estimates because of the high production costs of graphene and its complex use cases. Currently, the price of graphene ranges between $60-$200 per kg. Many experts believe that $10 per kg would change the world. But is it possible to source low-cost graphene?
Commonly, graphene layers are produced by exfoliating graphite ("top-down" method) using acids and high-energy electrochemical treatment, which usually leaves a defective perforated structure. Graphene can be made by stacking lattices from organic molecules by chemical vapor deposition ("bottom-up" method), which results in ultrasmall amounts with the highest prices.
There is a variety of methods to produce graphene, but none of them seems so close to the "low-cost graphene" goal as Rice University's novel (and still in its lab-phase state) flash graphene, created by using the Flash Joule Heating process in a custom-designed "flash reactor."
I'm sure the genius Beast from X-Men would like to hear more technical details, but the rest of us non-mutants are more interested in finding out "what's in it for me?"
But where does the plastic come from, anyway? If you thought about oil or natural gas, you're damn right! Fossil fuels are cracked into molecules for polymers using vast amounts of energy to create the heat and pressure needed.
The petrochemical industry accounts for more than 15% of global oil demand, and the growing demand for plastics, tires, clothing, fertilizers, and other everyday items will only increase that percentage. Consequently, the carbon-rich waste will only become a more significant problem.
Recycling plastic is a good idea in all those sustainability presentations and reports but poorly implemented in the real world. Greenpeace even claims that only 5% of plastic is actually recycled, while the OECD concluded that most of it ends up in landfills, is incinerated, or mismanaged.
It all comes down to costs. In many parts of the world, it's simply more costly to recycle plastics – just think about all the washing, sorting, and melting down of the plastics to turn them into a viable raw material. By comparison, creating plastic from fossil fuels is still cheaper (because related pollution is hardly taxed, but this is another story).
Flash Joule Heating could efficiently solve the problem if scaled up. In the lab, researchers used a mix of plastics without needing to sort or wash it. It was only grinded in small parts and then mixed with some iron and charcoal ash for conductivity.
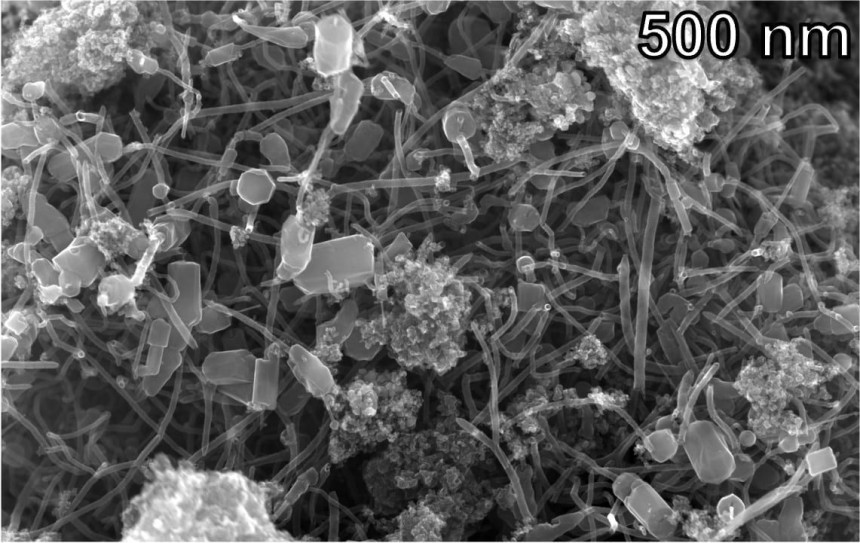
The Flash Joule Heating procedure consisted of a few tenth-of-a-second bursts of heat at temperatures over 5,120 °F / 2,820 °C. This way, researchers claim that they were able to obtain "a hybrid carbon nanomaterial that outperformed both graphene and commercially available carbon nanotubes."
These hybrid nanotubes allegedly improve the features of the composites they are combined with by a large margin. That's because graphene sheet bits attached to their end make composites much stronger and more durable.
Moreover, the life-cycle analysis of the entire process revealed that the Flash Joule Heating process uses 90% less energy and releases 94% less CO2 emissions than current methods for industrial carbon nanotube production.
If the industrial scale-up of this procedure will prove successful – and we're still in a "it's a big if" state – you can only imagine how lucrative the plastic recycling business will become. Turning carbon waste into highly valuable carbon nanotubes is like alchemists' dream of turning any metal into precious gold.
When they applied the Flash Joule Heating procedure to the mix of plastic waste in their "flash reactor," researchers also noticed all sorts of volatile gases. Using equipment to characterize the vaporized contents, they discovered that the Flash Joule Heating technique can recover two-thirds of the hydrogen in polyethylene "as a gas with a 94% purity."
In other words, "flashing" plastic waste not only results in valuable hybrid nanotubes and graphene but also clean hydrogen.
Currently, green hydrogen is sourced from splitting water with the help of electricity from renewables, but it's costly. Today, its price is around $5/kg, and experts believe that only in 2050 will we see a price drop to around $1/kg.
On the other hand, gray hydrogen is much cheaper – for instance, the one sourced by steam-methane reforming costs less than $1/kg. Unfortunately, it has a high environmental toll: for every ton of hydrogen, another 12 tons of CO2 are released into the atmosphere.
Remember that the industry uses nearly 100 million tons of hydrogen annually, and 95% is gray hydrogen. According to BloombergNEF, by 2050, the demand will increase five-fold, and all of it will need to be emissions-free for the net-zero emissions scenario.
Researchers at Rice University are convinced that their Flash Joule Heating process is an essential part of the solution. For now, they demonstrated – in the lab, don't forget it – that they can recover nearly 10% of the hydrogen in polyethylene, the most common plastic out there.
The world generates 350 to 400 million tons of plastic waste annually. To get an image, try and picture over 10 million garbage trucks discarding this amount in landfills, with too little of it being properly recycled.
Using Flash Joule Heating to recover even only 10% hydrogen from this tremendous amount of plastic waste would result in around 35 to 40 million tons of green hydrogen per year, much cheaper than splitting water and without the emissions of the gray hydrogen.
It's hard for me to understand why the Flash Joule Heating isn't already scaled up, why no billionaire or corporation has invested big in this idea, and why doesn't the whole world buzz about this "superhero" procedure.
Maybe this Flash Joule Heating thing is just a marketing stunt of Rice University to get funds. To date, this research has received less than $10 million only from the United States Army Engineer Research and Development Center, the Air Force Office of Scientific Research, and the National Science Foundation and the Office of Naval Research.
Or maybe investors wait to see more results and proof for a very profitable business case before pouring big money into this idea. One thing I'm sure of is that the Flash Joule Heating technique will eventually come true. I only hope this to happen sooner than too late.
What does the graphene have to do with the "Flash Joule Heating" technique?
Let me refresh your memory about this more than exciting process about which I wrote in a previous article. According to Rice University, using a 10-millisecond burst of light and heat of about 5,000 °F / 2,760 °C can turn any carbon-containing materials into graphene.The graphene is like a superhero material in the nanotechnology realm. Ok, Antman, please don't make a scene; we're not getting into Quantum Realm. Quantum mechanics describes aspects of nature at subatomic scales, while nanomaterials are on a near-atomic scale.
Graphene was discovered and scientifically proved in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester. They were later awarded the Nobel Prize in Physics 2010 for "groundbreaking experiments regarding the two-dimensional material graphene."
Wait, a 2-D material in a 3-D world? That's right, graphene is a one-atom-thick nanomaterial with exceptional tensile strength and electrical conductivity – just a few of its fascinating qualities suitable for electronics, energy, composites, or biomedicine.
The quest for low-cost graphene
You can learn much about graphene by googling it, but it's better to know more than school-level physics and chemistry. While it may seem not-so-complicated, graphene-based technologies are still very expensive. Still, in the last two decades, the interest in graphene has boomed.The graphene market is following the S-curve rule: while in 2012, its global market was valued at under $10 million / €8 million, ten years later, it jumped to around $700 million / €600 million, and the most recent forecasts estimate it will get to about $2.5 billion by 2028.
But get this: these are very conservative estimates because of the high production costs of graphene and its complex use cases. Currently, the price of graphene ranges between $60-$200 per kg. Many experts believe that $10 per kg would change the world. But is it possible to source low-cost graphene?
Commonly, graphene layers are produced by exfoliating graphite ("top-down" method) using acids and high-energy electrochemical treatment, which usually leaves a defective perforated structure. Graphene can be made by stacking lattices from organic molecules by chemical vapor deposition ("bottom-up" method), which results in ultrasmall amounts with the highest prices.
There is a variety of methods to produce graphene, but none of them seems so close to the "low-cost graphene" goal as Rice University's novel (and still in its lab-phase state) flash graphene, created by using the Flash Joule Heating process in a custom-designed "flash reactor."
How about getting rid of unwanted carbon waste?
Maybe you've heard the news: scientists discovered microplastics in the atmosphere. We already know it's in the water and even in fish. Now, apparently, we also breathe the plastic waste we created and disposed of in nature all these years.But where does the plastic come from, anyway? If you thought about oil or natural gas, you're damn right! Fossil fuels are cracked into molecules for polymers using vast amounts of energy to create the heat and pressure needed.
The petrochemical industry accounts for more than 15% of global oil demand, and the growing demand for plastics, tires, clothing, fertilizers, and other everyday items will only increase that percentage. Consequently, the carbon-rich waste will only become a more significant problem.
It all comes down to costs. In many parts of the world, it's simply more costly to recycle plastics – just think about all the washing, sorting, and melting down of the plastics to turn them into a viable raw material. By comparison, creating plastic from fossil fuels is still cheaper (because related pollution is hardly taxed, but this is another story).
Flash Joule Heating could efficiently solve the problem if scaled up. In the lab, researchers used a mix of plastics without needing to sort or wash it. It was only grinded in small parts and then mixed with some iron and charcoal ash for conductivity.
The Flash Joule Heating procedure consisted of a few tenth-of-a-second bursts of heat at temperatures over 5,120 °F / 2,820 °C. This way, researchers claim that they were able to obtain "a hybrid carbon nanomaterial that outperformed both graphene and commercially available carbon nanotubes."
These hybrid nanotubes allegedly improve the features of the composites they are combined with by a large margin. That's because graphene sheet bits attached to their end make composites much stronger and more durable.
Moreover, the life-cycle analysis of the entire process revealed that the Flash Joule Heating process uses 90% less energy and releases 94% less CO2 emissions than current methods for industrial carbon nanotube production.
Bonus: the unexpected by-product of "flashing" plastic waste
Well, now, don't get any time-traveling ideas like in The Flash comics because the byproduct I'm talking about is only the most abundant chemical element in the Universe: Hydrogen. That's right, Rice University researchers dare to claim they can source low-cost 100% green hydrogen from carbon-rich plastic waste.When they applied the Flash Joule Heating procedure to the mix of plastic waste in their "flash reactor," researchers also noticed all sorts of volatile gases. Using equipment to characterize the vaporized contents, they discovered that the Flash Joule Heating technique can recover two-thirds of the hydrogen in polyethylene "as a gas with a 94% purity."
In other words, "flashing" plastic waste not only results in valuable hybrid nanotubes and graphene but also clean hydrogen.
Currently, green hydrogen is sourced from splitting water with the help of electricity from renewables, but it's costly. Today, its price is around $5/kg, and experts believe that only in 2050 will we see a price drop to around $1/kg.
On the other hand, gray hydrogen is much cheaper – for instance, the one sourced by steam-methane reforming costs less than $1/kg. Unfortunately, it has a high environmental toll: for every ton of hydrogen, another 12 tons of CO2 are released into the atmosphere.
Remember that the industry uses nearly 100 million tons of hydrogen annually, and 95% is gray hydrogen. According to BloombergNEF, by 2050, the demand will increase five-fold, and all of it will need to be emissions-free for the net-zero emissions scenario.
The world generates 350 to 400 million tons of plastic waste annually. To get an image, try and picture over 10 million garbage trucks discarding this amount in landfills, with too little of it being properly recycled.
Using Flash Joule Heating to recover even only 10% hydrogen from this tremendous amount of plastic waste would result in around 35 to 40 million tons of green hydrogen per year, much cheaper than splitting water and without the emissions of the gray hydrogen.
Too good to be true. But maybe I'm wrong
Theoretically, "flashing" the plastic waste discarded in the last decades would already provide humanity with hundreds of millions of emission-free hydrogen. At the same time, high-tech industries would benefit from low-cost carbon nanotubes and valuable graphene.It's hard for me to understand why the Flash Joule Heating isn't already scaled up, why no billionaire or corporation has invested big in this idea, and why doesn't the whole world buzz about this "superhero" procedure.
Maybe this Flash Joule Heating thing is just a marketing stunt of Rice University to get funds. To date, this research has received less than $10 million only from the United States Army Engineer Research and Development Center, the Air Force Office of Scientific Research, and the National Science Foundation and the Office of Naval Research.
Or maybe investors wait to see more results and proof for a very profitable business case before pouring big money into this idea. One thing I'm sure of is that the Flash Joule Heating technique will eventually come true. I only hope this to happen sooner than too late.