New advances in sodium-ion battery technology might finally pave the way toward replacing the ubiquitous lithium-ion batteries. The latest research covers revolutionary solid electrolytes and cathode materials that will turn sodium-ion batteries into high-density, fast-charging alternatives to current Li-ion cells.
Lithium-ion batteries power almost every portable device on Earth, from smartwatches and drones to smartphones and laptops. They are also critical to the success of electric vehicles and energy storage systems, ranging from home backup systems to utility-scale storage batteries. Lithium-ion batteries are a fairly new technology, with the first batteries being sold in 1991 after more than 35 years of research and development.
The technology has vastly improved over the years. Li-ion cells are relatively cheap and easy to manufacture, have high energy density, and have a long lifecycle. However, depending on the chemistry, they can be thermally unstable. This might not be a problem for small batteries that use one or two cells, which are easy to cool. However, when hundreds or even thousands of them are installed inside a battery pack, they need good thermal management systems to keep them from overheating. When this happens, they can burst into flames, a process called "thermal runaway."
This is why researchers are always trying to find new electrolytes and chemistries that offer improvements in areas where Li-ion batteries are not very good. However, there are always trade-offs with Li-ion batteries, which means improving something without ruining other characteristics is impossible. For instance, lithium-iron-phosphate batteries, the latest craze in Li-ion battery tech, are safer and offer much longer lifecycles than other Li-ion chemistries. However, they have much lower energy density and charge slower, especially in the cold.
Li-ion batteries also have the disadvantage that the most performant types use critical raw materials that are expensive, hard to find, or difficult to process. This is why LFP cells have become so popular despite their shortcomings. These cells use iron instead of nickel and cobalt, which makes them cheaper and more environmentally friendly than nickel-based batteries. However, researchers think there are better battery types than LFP, even though they can't yet compete with Li-ion batteries.
Major efforts are made toward developing solid electrolytes instead of the current liquid types. These can theoretically offer much higher energy densities and are safer because they are non-flammable. However, since batteries expand and contract during charging and discharging, they develop cracks that allow dendrites to grow between electrodes. Sooner rather than later, the dendrites short-circuit the electrodes, destroying the cells. This issue has been bothering researchers for years, this being one of the last barriers to commercial solid-state batteries.
Other research teams have focused on finding new materials with better properties than those currently used in Li-ion batteries. Although lithium seems abundant, it is still challenging to mine and process. Sodium, on the other hand, is found everywhere and is much cheaper while sharing many properties with lithium. This is why sodium-ion batteries have been a natural development in the quest to replace Li-ion batteries with something better or at least cheaper.
Sodium-ion batteries have many advantages, but perhaps the most important is that they can be manufactured using the same industrial processes as their lithium-based counterparts. Other benefits include increased safety and the ability to be discharged to zero volts without affecting their integrity. Major downsides are lower energy density, even compared to the least performant LFP cells, and the long charging times.
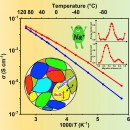
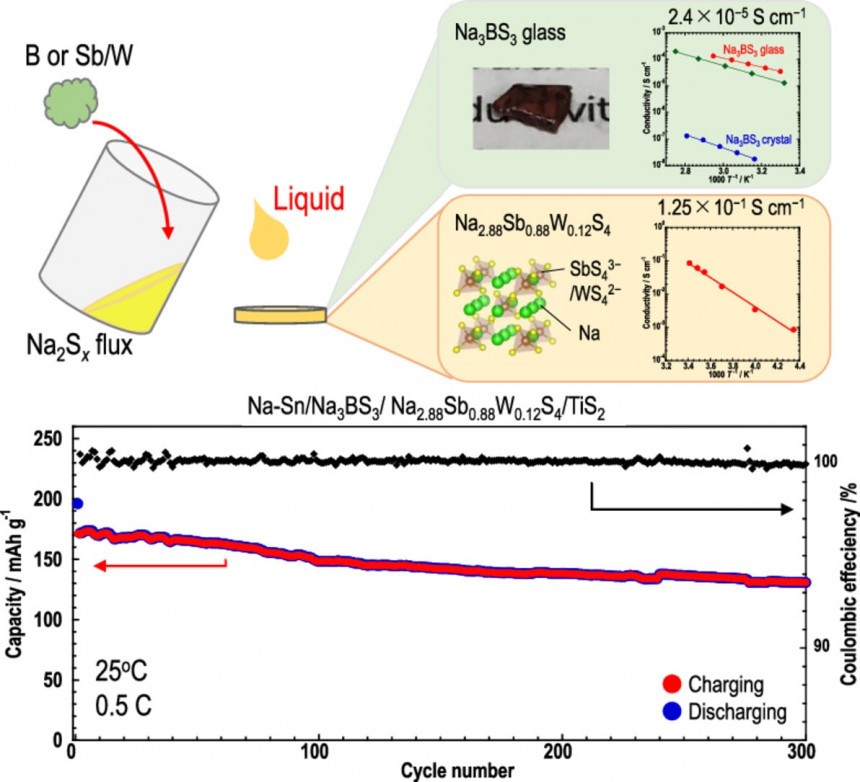
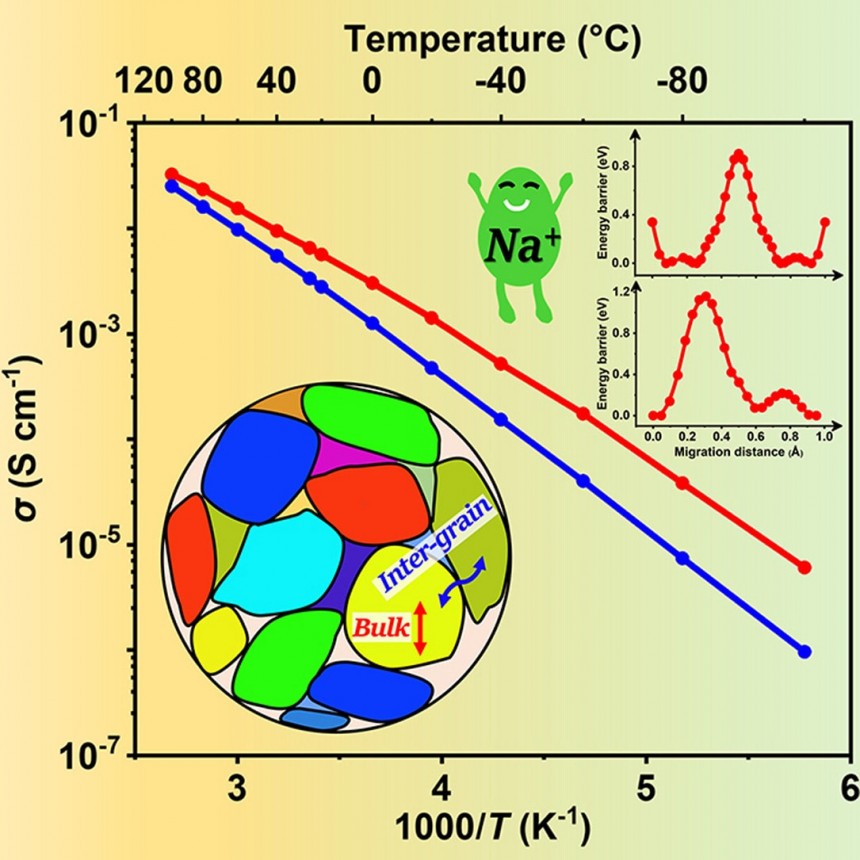
However, recent advances promise to eliminate these issues and make sodium-ion batteries the go-to solution for replacing Li-ion cells. Last month, researchers at the Osaka Metropolitan University in Japan announced they developed a mass synthesis process for high-conductivity electrolytes in solid-state sodium batteries. Their mass synthesis process yields solid sulfide electrolytes with the world's highest reported sodium ion conductivity. It also created glass electrolytes with high formability, allowing them to deform without developing cracks.
The newly developed process is useful for producing almost all sodium-containing sulfide materials, including solid electrolytes and electrode active materials. Osaka Metropolitan University Associate Professor Atsushi Sakuda believes this will become a mainstream process for developing future materials for all-solid-state sodium batteries.
Around the same time, a team at the Karlsruhe Institute of Technology in Germany developed a groundbreaking solid electrolyte that dramatically enhances battery performance at room temperature. The electrolyte, called NYZS because it contains Sodium (Na), Yttrium (Y), Zirconium (Zr), and Silicon (Si), enhances the efficient conduction of ions at room temperature, which is crucial for practical energy-storage applications. The NYZS electrolyte exhibits high ionic conductivity and demonstrates remarkable electrochemical stability, withstanding voltages over 10 volts.
However, a team at the Korea Advanced Institute of Science and Technology (KAIST) may have made a bigger discovery that will vastly improve charging speeds. The KAIM team replaced common battery cathode materials with those used by supercapacitors, creating a high-energy, high-output hybrid sodium-ion battery capable of rapid charging.
The hybrid system has the advantage of having high storage capacity and fast charge and discharge speed by combining the battery cathode materials and the capacitor anode materials. However, the biggest challenge was to improve the relatively slow energy storage speed of the battery cathode alongside the lower energy storage capacity of the capacitor anode material. The result was a sodium-ion battery with a high energy density of 247 Wh/kg and ultra-fast charging capabilities, suitable for use in electric vehicles.
The technology has vastly improved over the years. Li-ion cells are relatively cheap and easy to manufacture, have high energy density, and have a long lifecycle. However, depending on the chemistry, they can be thermally unstable. This might not be a problem for small batteries that use one or two cells, which are easy to cool. However, when hundreds or even thousands of them are installed inside a battery pack, they need good thermal management systems to keep them from overheating. When this happens, they can burst into flames, a process called "thermal runaway."
This is why researchers are always trying to find new electrolytes and chemistries that offer improvements in areas where Li-ion batteries are not very good. However, there are always trade-offs with Li-ion batteries, which means improving something without ruining other characteristics is impossible. For instance, lithium-iron-phosphate batteries, the latest craze in Li-ion battery tech, are safer and offer much longer lifecycles than other Li-ion chemistries. However, they have much lower energy density and charge slower, especially in the cold.
Li-ion batteries also have the disadvantage that the most performant types use critical raw materials that are expensive, hard to find, or difficult to process. This is why LFP cells have become so popular despite their shortcomings. These cells use iron instead of nickel and cobalt, which makes them cheaper and more environmentally friendly than nickel-based batteries. However, researchers think there are better battery types than LFP, even though they can't yet compete with Li-ion batteries.
Other research teams have focused on finding new materials with better properties than those currently used in Li-ion batteries. Although lithium seems abundant, it is still challenging to mine and process. Sodium, on the other hand, is found everywhere and is much cheaper while sharing many properties with lithium. This is why sodium-ion batteries have been a natural development in the quest to replace Li-ion batteries with something better or at least cheaper.
Sodium-ion batteries have many advantages, but perhaps the most important is that they can be manufactured using the same industrial processes as their lithium-based counterparts. Other benefits include increased safety and the ability to be discharged to zero volts without affecting their integrity. Major downsides are lower energy density, even compared to the least performant LFP cells, and the long charging times.
However, recent advances promise to eliminate these issues and make sodium-ion batteries the go-to solution for replacing Li-ion cells. Last month, researchers at the Osaka Metropolitan University in Japan announced they developed a mass synthesis process for high-conductivity electrolytes in solid-state sodium batteries. Their mass synthesis process yields solid sulfide electrolytes with the world's highest reported sodium ion conductivity. It also created glass electrolytes with high formability, allowing them to deform without developing cracks.
Around the same time, a team at the Karlsruhe Institute of Technology in Germany developed a groundbreaking solid electrolyte that dramatically enhances battery performance at room temperature. The electrolyte, called NYZS because it contains Sodium (Na), Yttrium (Y), Zirconium (Zr), and Silicon (Si), enhances the efficient conduction of ions at room temperature, which is crucial for practical energy-storage applications. The NYZS electrolyte exhibits high ionic conductivity and demonstrates remarkable electrochemical stability, withstanding voltages over 10 volts.
However, a team at the Korea Advanced Institute of Science and Technology (KAIST) may have made a bigger discovery that will vastly improve charging speeds. The KAIM team replaced common battery cathode materials with those used by supercapacitors, creating a high-energy, high-output hybrid sodium-ion battery capable of rapid charging.
The hybrid system has the advantage of having high storage capacity and fast charge and discharge speed by combining the battery cathode materials and the capacitor anode materials. However, the biggest challenge was to improve the relatively slow energy storage speed of the battery cathode alongside the lower energy storage capacity of the capacitor anode material. The result was a sodium-ion battery with a high energy density of 247 Wh/kg and ultra-fast charging capabilities, suitable for use in electric vehicles.