Synthetic fuels are a great way not only to use the carbon dioxide captured from the air but also to extend the life of internal combustion engines. Up until now, turning carbon dioxide into fuels was considered a costly and unpractical solution, but a breakthrough discovery helped increase the efficiency to make eFuels a viable solution.
We’ve seen several attempts to produce synthetic fuels using the carbon dioxide captured from the air. Porsche and Audi were among the brands that went the furthest on this road, with the former even considering opening an eFuel plant in Chile. But the main problem is converting carbon dioxide into fuel is a very energy-consuming process. The efficiency is also low, which questions the viability of synthetic fuels.
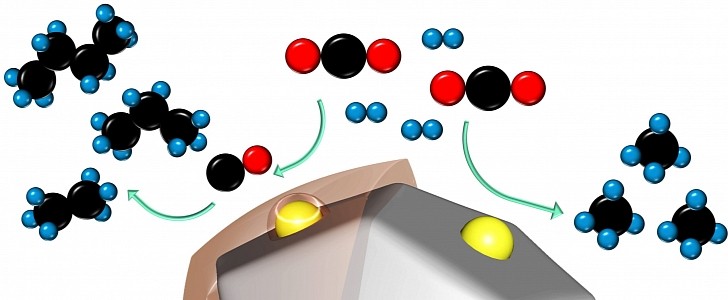
Thanks to a breakthrough catalyst developed by chemical engineers at Stanford University, this will dramatically change soon. The technology turns waste CO2 and hydrogen into chains of methane, propane, and even butane, all of them usable as a fuel source. The process is nothing new, but the scale and efficiency that this discovery can achieve is a game-changer.
“We can create gasoline, basically,” says Matteo Cargnello, a chemical engineer at Stanford University. “To capture as much carbon as possible, you want the longest chain hydrocarbons. Chains with eight to 12 carbon atoms would be the ideal.”
The current technologies can achieve this but need extremely high temperatures and pressure that make the whole process quite expensive. Cargnello and his team found out that using a ruthenium catalyst covered with a special organic polymer would maximize the reactivity of carbon monoxide and hydrogen. The efficiency ramped up 1,000-fold in the case of four-carbon-long chains of butane.
Although turning carbon dioxide into fuel sounds like a promising possibility, the goal is getting to longer carbon chains that are more stable than the gaseous fuels. This includes gasoline, but also non-fuels like olefins used to make plastic. This way the carbon will remain trapped for a long time, paving the way for a significant reduction in the carbon dioxide levels in the atmosphere.
Thanks to a breakthrough catalyst developed by chemical engineers at Stanford University, this will dramatically change soon. The technology turns waste CO2 and hydrogen into chains of methane, propane, and even butane, all of them usable as a fuel source. The process is nothing new, but the scale and efficiency that this discovery can achieve is a game-changer.
“We can create gasoline, basically,” says Matteo Cargnello, a chemical engineer at Stanford University. “To capture as much carbon as possible, you want the longest chain hydrocarbons. Chains with eight to 12 carbon atoms would be the ideal.”
The current technologies can achieve this but need extremely high temperatures and pressure that make the whole process quite expensive. Cargnello and his team found out that using a ruthenium catalyst covered with a special organic polymer would maximize the reactivity of carbon monoxide and hydrogen. The efficiency ramped up 1,000-fold in the case of four-carbon-long chains of butane.
Although turning carbon dioxide into fuel sounds like a promising possibility, the goal is getting to longer carbon chains that are more stable than the gaseous fuels. This includes gasoline, but also non-fuels like olefins used to make plastic. This way the carbon will remain trapped for a long time, paving the way for a significant reduction in the carbon dioxide levels in the atmosphere.