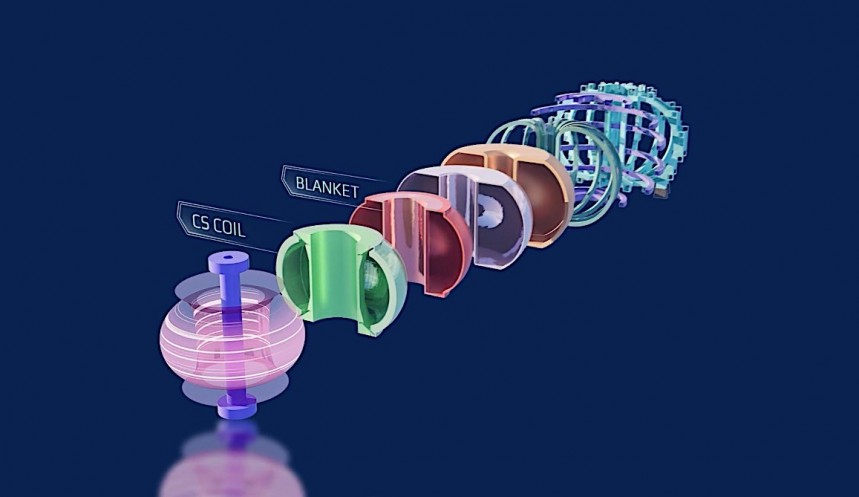
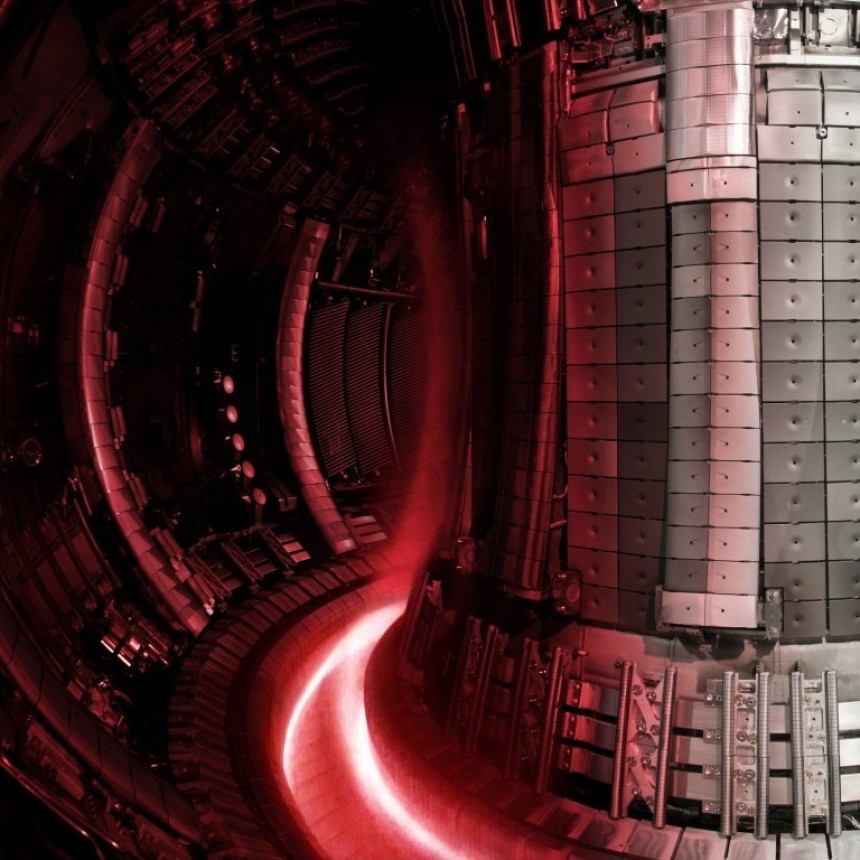
After the National Ignition Facility in California's first successful scientific breakeven through nuclear fusion, mankind is all the more serious about reaching the promised land of near-limitless fusion energy. Perhaps the most pressing problem at this stage is that commercial-scale fusion will no doubt need a lot of fuel. That's why understanding the duality between nuclear fusion and fission is nothing short of crucial.
It's easy to forget that nuclear fusion and fission are two sides of the same coin. One may conjure images of meltdowns, radiation sickness, and general nightmare fuel. While the other is mostly in the realm of science fiction. At least, it was all sci-fi until recently.
If there were a single atom you could point to as encapsulating the yin and yang-like parallels between nuclear fission and fusion, look no further than tritium. It's an isotope of hydrogen so rare in nature on Earth that it's almost unfair humanity discovered its considerable usefulness. It's all the sadder because tritium is a bonafide superstar in nuclear fusion.
For all the petrolheads reading this, various types of nuclear fusion isotopes have much more in common with different types of gasoline than you might think. If scientists are so gung-ho about amassing as big of a global tritium stockpile as possible, it's a signifier the isotope isn't just any old 87-octane. It's the real premium, top-tier stuff.
But why? What is it about tritium that makes it so irresistible to fusion engineers? Despite its important-sounding, sci-fi adjacent name, tritium is just another, albeit a particularly "spicy" and radioactive isotope of old-fashioned hydrogen. Unlike standard hydrogen, tritium contains one proton and two neutrons to the standard one proton without neutrons.
Along with its sibling isotope of deuterium with one proton and one neutron, isotopes of hydrogen are the only ones to receive their own nomenclature as if they were separate elements. First discovered in 1934 by Ernest Rutherford, Mark Oliphant, and Paul Harteck, tritium was first isolated five years later by the American physicist Luis Walter Alvarez.
In the early days, tritium's practical use for mankind was the radiometric dating of liquids, usually water or wine. Alternatively, small quantities of tritium can be added to tiny glass tubes to create glow-in-the-dark wristwatches that stay luminescent for at least a decade, if not more, depending on the purity.
Tritium isn't as overtly dangerous as other luminescent wristwatch materials like radium. Which famously made the jaws of the workers making the watches fall off after dipping their paintbrushes in the stuff and then in their mouths to point the brush tip. It'd take a little longer to understand this astonishing isotope's full energy potential. In truth, tritium was more closely studied in relation to its use in thermonuclear bombs before it was pegged as the future of nuclear fusion fuel.
An idea for potentially adding solid lithium-deuteride to the fission stage of a hydrogen bomb to form tritium was floated at one point by the U.S.. All in the name of increasing the explosive power in the fusion stage without making the weapon physically larger were had during the Cold War. Among the explosive thermonuclear reaction's elemental leftovers is a non-unsubstantial amount of residual tritium.
But uncontrolled nuclear explosions are perhaps the least constructive application of tritium. To make one more gasoline analogy, tritium isn't just your average premium 93-octane. Instead, its rarity and horsepower potential are more like 110-octane race gas. You know, the stuff that maybe a handful of gas stations in your tri-state area carry and never located anywhere near you.
The energy generated by deuterium and tritium fusion, in particular, far exceeds other combinations of isotopes. The tritium-deuterium fusion process, should it succeed as planned, should generate an entirely new single atom of helium plus a spare neutron left over. Per every set of atoms fused, each tritium-deuterium fusion should generate at least 17.6 electron volts (MeV) per reaction.
Compared to other forms of fusion like deuterium-to-deuterium or even deuterium-to-helium-3, the energy release is profoundly more intense. It sounds like a win-win scenario, apart from one massive problem. You could fit the entire planet's reserves of tritium into a slightly above-average-sized suitcase. Only about 44 lbs (20 kg) is known to be in reserve in all the world's facilities. That's not including the plethora of fake tritium watchmakers passing them off as tritium when it really isn't.
Fusion reactors that rely heavily on tritium, like the Tokamak reactors at the JET Laboratory in the U.K. and the ITER facility in France, could eat through 300 grams of the stuff every single day in commercial operation. Happily, it's possible to breed new tritium in the same vein as farming XP in a video game. Meaning, of course, very slowly and at a great expense of time.
The most popular form of what's called "tritium breeding" occurs as a byproduct of, of all things, nuclear fission reactors. More specifically, the fission of uranium isotopes like 235 and 233, but also plutonium-239. This process is most prevalent in light-water or heavy-water cooled fission reactors like the Canadian CANDU-class are known to produce as much as 4.6 ounces (130 grams) of tritium per reactor per year.
With a total of 31 active CANDU reactors in Canada, South Korea, China, India, Argentina, Pakistan, and Romania, humanity still struggles to produce enough tritium to run the average Tokamak-class fusion reactor for more than a couple of months. The U.S.'s 56 pressurized water and 24 boiling water reactors produce tritium as an after product, but can only produce enough of it to equal around 4.2 grams as of 2023.
The EPA estimates that by 2030, global man-made supplies of tritium will be equal the amount estimated to exist in nature. Imagine that happening with literally any other element, that's absolutely bonkers! Keep in mind not all the tritium fission reactors produce is able to be harvested. Several American fission reactors have had tritium escape containment and confirmed by the EPA. In one case, this left the surrounding groundwater contaminated with 7.5 microcuries (280 kBq) of tritium per liter. That's almost 400 times the World Health Organization's recommended limit.
France's ITER facility Tokamak generator does plan to bombard pieces of lithium metal with some of the neutrons flung off from fusion plasma to help further boost tritium stockpiles. But the efficacy of this has yet to be proven. Tritium can also be found in exceedingly scarce quantities at the very fringes of Earth's atmosphere. Here, cosmic rays from the Sun interact with nitrogen molecules not fortunate enough to be shielded by the bulk of the atmosphere beneath it to create tritium gas.
Not very beneficial for fusion production, that's for sure. Considering tritium has a half-life decay of around 12 years or so, reserves can and will decline if we don't use it quickly enough. Compared to deuterium, which makes up about one in every 5,000 hydrogen atoms in most seawater, it's a shame we can't just fuse deuterium with itself till the cows come home.
But as private fusion research companies like Helion Energy have found, deuterium-to-deuterium fusion produces only a fraction of the energy as deuterium-to-tritium vision. All while potentially damaging the reactor while it attempts nuclear fusion in excess of 100 million degrees.
Considering tritium naturally decays into helium-3, another staple isotope of nuclear fusion research, some argue it's better to skip tritium altogether and just fuse deuterium and helium-3 into helium-4 plus an extra atom of hydrogen. The sad truth of the matter is that scientists are forced to essentially throw cupcakes at a wall to see which ones stick the longest when it comes to finding the right mix of fusion fuels. I.e., nothing more than old-fashioned trial and error.
With so many private companies developing their own novel fusion methods, one combination should prove to be the ticket before too long. The only question is whether tritium will or won't be included in the recipe when all said and done. Whichever team manages to figure it out and make it viable enough for commercial operation is due to be rich beyond most people's wildest imagination. That's on top of all the other added benefits of nearly limitless, carbon-free energy.
If there were a single atom you could point to as encapsulating the yin and yang-like parallels between nuclear fission and fusion, look no further than tritium. It's an isotope of hydrogen so rare in nature on Earth that it's almost unfair humanity discovered its considerable usefulness. It's all the sadder because tritium is a bonafide superstar in nuclear fusion.
For all the petrolheads reading this, various types of nuclear fusion isotopes have much more in common with different types of gasoline than you might think. If scientists are so gung-ho about amassing as big of a global tritium stockpile as possible, it's a signifier the isotope isn't just any old 87-octane. It's the real premium, top-tier stuff.
But why? What is it about tritium that makes it so irresistible to fusion engineers? Despite its important-sounding, sci-fi adjacent name, tritium is just another, albeit a particularly "spicy" and radioactive isotope of old-fashioned hydrogen. Unlike standard hydrogen, tritium contains one proton and two neutrons to the standard one proton without neutrons.
In the early days, tritium's practical use for mankind was the radiometric dating of liquids, usually water or wine. Alternatively, small quantities of tritium can be added to tiny glass tubes to create glow-in-the-dark wristwatches that stay luminescent for at least a decade, if not more, depending on the purity.
Tritium isn't as overtly dangerous as other luminescent wristwatch materials like radium. Which famously made the jaws of the workers making the watches fall off after dipping their paintbrushes in the stuff and then in their mouths to point the brush tip. It'd take a little longer to understand this astonishing isotope's full energy potential. In truth, tritium was more closely studied in relation to its use in thermonuclear bombs before it was pegged as the future of nuclear fusion fuel.
An idea for potentially adding solid lithium-deuteride to the fission stage of a hydrogen bomb to form tritium was floated at one point by the U.S.. All in the name of increasing the explosive power in the fusion stage without making the weapon physically larger were had during the Cold War. Among the explosive thermonuclear reaction's elemental leftovers is a non-unsubstantial amount of residual tritium.
The energy generated by deuterium and tritium fusion, in particular, far exceeds other combinations of isotopes. The tritium-deuterium fusion process, should it succeed as planned, should generate an entirely new single atom of helium plus a spare neutron left over. Per every set of atoms fused, each tritium-deuterium fusion should generate at least 17.6 electron volts (MeV) per reaction.
Compared to other forms of fusion like deuterium-to-deuterium or even deuterium-to-helium-3, the energy release is profoundly more intense. It sounds like a win-win scenario, apart from one massive problem. You could fit the entire planet's reserves of tritium into a slightly above-average-sized suitcase. Only about 44 lbs (20 kg) is known to be in reserve in all the world's facilities. That's not including the plethora of fake tritium watchmakers passing them off as tritium when it really isn't.
Fusion reactors that rely heavily on tritium, like the Tokamak reactors at the JET Laboratory in the U.K. and the ITER facility in France, could eat through 300 grams of the stuff every single day in commercial operation. Happily, it's possible to breed new tritium in the same vein as farming XP in a video game. Meaning, of course, very slowly and at a great expense of time.
With a total of 31 active CANDU reactors in Canada, South Korea, China, India, Argentina, Pakistan, and Romania, humanity still struggles to produce enough tritium to run the average Tokamak-class fusion reactor for more than a couple of months. The U.S.'s 56 pressurized water and 24 boiling water reactors produce tritium as an after product, but can only produce enough of it to equal around 4.2 grams as of 2023.
The EPA estimates that by 2030, global man-made supplies of tritium will be equal the amount estimated to exist in nature. Imagine that happening with literally any other element, that's absolutely bonkers! Keep in mind not all the tritium fission reactors produce is able to be harvested. Several American fission reactors have had tritium escape containment and confirmed by the EPA. In one case, this left the surrounding groundwater contaminated with 7.5 microcuries (280 kBq) of tritium per liter. That's almost 400 times the World Health Organization's recommended limit.
France's ITER facility Tokamak generator does plan to bombard pieces of lithium metal with some of the neutrons flung off from fusion plasma to help further boost tritium stockpiles. But the efficacy of this has yet to be proven. Tritium can also be found in exceedingly scarce quantities at the very fringes of Earth's atmosphere. Here, cosmic rays from the Sun interact with nitrogen molecules not fortunate enough to be shielded by the bulk of the atmosphere beneath it to create tritium gas.
But as private fusion research companies like Helion Energy have found, deuterium-to-deuterium fusion produces only a fraction of the energy as deuterium-to-tritium vision. All while potentially damaging the reactor while it attempts nuclear fusion in excess of 100 million degrees.
Considering tritium naturally decays into helium-3, another staple isotope of nuclear fusion research, some argue it's better to skip tritium altogether and just fuse deuterium and helium-3 into helium-4 plus an extra atom of hydrogen. The sad truth of the matter is that scientists are forced to essentially throw cupcakes at a wall to see which ones stick the longest when it comes to finding the right mix of fusion fuels. I.e., nothing more than old-fashioned trial and error.
With so many private companies developing their own novel fusion methods, one combination should prove to be the ticket before too long. The only question is whether tritium will or won't be included in the recipe when all said and done. Whichever team manages to figure it out and make it viable enough for commercial operation is due to be rich beyond most people's wildest imagination. That's on top of all the other added benefits of nearly limitless, carbon-free energy.