With so many cars on public streets, concerns that the Planet might run out of petrol are becoming more of a common thing nowadays, although vehicles aren't exactly the biggest burners out there. However, efforts to replace gasoline as the main way to put a car in motion have received the support of authorities and organizations from all over the world. In the last couple of years, most companies focused their efforts on electric motors and hybrid engines, briefly known as green cars, but even so, their adoption might take a while.
Renault for example tried to secure partnerships in every country across the globe not only to be sure that it will be able to sell its cars there, but also to encourage customers to replace their traditional gasoline-powered models with electric ways of propulsion.
Either way, getting rid of gasoline, or at least, cutting fuel consumption and minimizing emissions might have a simpler and less expensive solution. Water, that is. There are hundreds, maybe thousands of inventors who tried to patent their very own way to put a car in motion using water but, until now, no carmaker has moved towards a mass production process of such a prototype. In fact, there are several aftermarket kits out there, but a factory-installed product is yet to reach the mass market.
WHAT THEY ACTUALLY ARE
Before jumping into details, we must clarify a major issue. Cars that work with water weren't, aren't and will most likely not be 100 percent based on water. This means that water cannot be the only "fuel" inside a vehicle and it only works in conjunction with gasoline. And why such a big deal? you might ask. Well, because using water as an alternative fuel would pretty much mean reduced fuel consumption and less emissions, although critics beg to differ.
In just a few words, there are a few voices out there that believe that installing a pre-made conversion kit on a car isn't such a big deal because the whole system would need almost more energy to split the water and conduct the whole process as a regular combustion engine. Moreover, some are even claiming that this entire assembly might have a negative impact on fuel efficiency, reducing it rather than improving.
WATER AS A MEANS OF PROPULSION
In order to be used as "fuel" (we're using quotation marks to avoid a confusion, water IS NOT fuel), the conversion kit we were talking about must act as an electrolyzer. This actually means that water must be split in the two elements that form it, namely hydrogen and oxygen. The whole reaction is pretty simple and is backed by a separate pack of batteries or straight by the car's alternator, but the second scenario might overuse the alternative and lead to a number of problems.
The two are then injected into the engine and mixed with gasoline, with the mixture bearing the name of oxyhydrogen or "Brown's gas", after Yull Brown has put the bases of devices using it. The compound is, as we said, pumped into the engine where it is mixed with gasoline and air. The result? More energy and less emissions, as the engine needs less gasoline to provide power, hydrogen playing a key role in the whole process.
Since the amount of energy is over the level the engine needs to achieve to put the car in motion and generate the necessary power, a certain percentage can also be re-transferred into the conversion kit where it could be used to electrolyze more water. The chemical reaction of the electrolysis is the following:
2H2O → 2H2 + O2
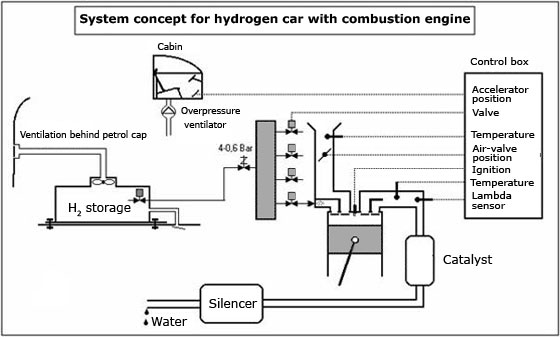
Although they might sound similar, water-propelled cars are different from hydrogen cars. In the latter, the car is directly pumped and stored inside a tank, just like regular gasoline, while in water-fueled vehicles, the hydrogen is obtained after a chemical reaction.
FACTS AND FIGURES
There are several prototypes out there, more or less attention worthy, but all of them having the same goal: cut fuel consumption and reduce emissions. But, after all, how efficient can such an application actually be?
Well, numbers are varying a lot, but a recent project set up by a 19-year old student proves that fuel efficiency can be increased by up to 30 percent. The modifications were applied on an 1997 Elantra and used 12V energy from the battery to separate hydrogen and oxygen from drinking water.
It is generally believed that such a technology could cut fuel consumption by up to 50 percent, but real figures are obviously less shocking. Furthermore, online ads and promotional campaigns claim that engine power will be "greatly" improved but, once again, reality is pretty different and the amount of power won't be that big unless you're ready to do some "not recommended" changes to the way the system works and risk serious engine damage.
Renault for example tried to secure partnerships in every country across the globe not only to be sure that it will be able to sell its cars there, but also to encourage customers to replace their traditional gasoline-powered models with electric ways of propulsion.
Either way, getting rid of gasoline, or at least, cutting fuel consumption and minimizing emissions might have a simpler and less expensive solution. Water, that is. There are hundreds, maybe thousands of inventors who tried to patent their very own way to put a car in motion using water but, until now, no carmaker has moved towards a mass production process of such a prototype. In fact, there are several aftermarket kits out there, but a factory-installed product is yet to reach the mass market.
WHAT THEY ACTUALLY ARE
Before jumping into details, we must clarify a major issue. Cars that work with water weren't, aren't and will most likely not be 100 percent based on water. This means that water cannot be the only "fuel" inside a vehicle and it only works in conjunction with gasoline. And why such a big deal? you might ask. Well, because using water as an alternative fuel would pretty much mean reduced fuel consumption and less emissions, although critics beg to differ.
In just a few words, there are a few voices out there that believe that installing a pre-made conversion kit on a car isn't such a big deal because the whole system would need almost more energy to split the water and conduct the whole process as a regular combustion engine. Moreover, some are even claiming that this entire assembly might have a negative impact on fuel efficiency, reducing it rather than improving.
WATER AS A MEANS OF PROPULSION
In order to be used as "fuel" (we're using quotation marks to avoid a confusion, water IS NOT fuel), the conversion kit we were talking about must act as an electrolyzer. This actually means that water must be split in the two elements that form it, namely hydrogen and oxygen. The whole reaction is pretty simple and is backed by a separate pack of batteries or straight by the car's alternator, but the second scenario might overuse the alternative and lead to a number of problems.
The two are then injected into the engine and mixed with gasoline, with the mixture bearing the name of oxyhydrogen or "Brown's gas", after Yull Brown has put the bases of devices using it. The compound is, as we said, pumped into the engine where it is mixed with gasoline and air. The result? More energy and less emissions, as the engine needs less gasoline to provide power, hydrogen playing a key role in the whole process.
Since the amount of energy is over the level the engine needs to achieve to put the car in motion and generate the necessary power, a certain percentage can also be re-transferred into the conversion kit where it could be used to electrolyze more water. The chemical reaction of the electrolysis is the following:
2H2O → 2H2 + O2
Although they might sound similar, water-propelled cars are different from hydrogen cars. In the latter, the car is directly pumped and stored inside a tank, just like regular gasoline, while in water-fueled vehicles, the hydrogen is obtained after a chemical reaction.
FACTS AND FIGURES
There are several prototypes out there, more or less attention worthy, but all of them having the same goal: cut fuel consumption and reduce emissions. But, after all, how efficient can such an application actually be?
Well, numbers are varying a lot, but a recent project set up by a 19-year old student proves that fuel efficiency can be increased by up to 30 percent. The modifications were applied on an 1997 Elantra and used 12V energy from the battery to separate hydrogen and oxygen from drinking water.
It is generally believed that such a technology could cut fuel consumption by up to 50 percent, but real figures are obviously less shocking. Furthermore, online ads and promotional campaigns claim that engine power will be "greatly" improved but, once again, reality is pretty different and the amount of power won't be that big unless you're ready to do some "not recommended" changes to the way the system works and risk serious engine damage.