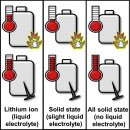
The automotive industry is eagerly waiting for solid-state batteries to become commercially viable. A breakthrough is just around the corner, everybody says, and this means higher energy density and increased safety for our electric vehicles. About that safety, though, it might not materialize, warns a new study.
There’s not a single day that we don’t find about new developments in the solid-state batteries’ space but that “breakthrough” does not happen. Rome wasn’t built in a day, though, and sometimes big advances happen in small steps. Solid-state batteries are said to come with big advantages, like higher energy density than those based on liquid electrolytes. For now, though, they seem to have more drawbacks than advantages, which makes them unsuitable for commercial applications.
There’s a long-time belief that solid electrolytes make the batteries safer because they are nonflammable. It may be that they are a lot more stable than their counterparts, but a new study shows this does not necessarily make them safer. According to the U.S. DOE Sandia National Laboratories, solid electrolytes can still fail under certain conditions. This happens when the battery is crushed, punctured or when the internal pressure builds up to a level that makes the lithium react with the internal oxygen.
Regardless of whether they have a solid or liquid electrolyte, all Li-Ion batteries form dendrite spikes under normal use. They have the bad habit of puncturing through the layers of separator in the battery, causing a short circuit. When the solid electrolyte does fail, the resulting short circuit will lead to the same heat build-up as in the case of a conventional Li-Ion battery.
In some cases, short-circuited solid-state batteries can reach temperatures significantly higher than conventional Li-ion, shows the Sandia study published in the scientific journal Joule. This happens because there’s a huge amount of energy stored in the battery that gets released when the positive and the negative poles are no longer separated by the electrolyte. There’s no way around that, and although the electrolyte is nonflammable, other surrounding materials might be.
There’s a long-time belief that solid electrolytes make the batteries safer because they are nonflammable. It may be that they are a lot more stable than their counterparts, but a new study shows this does not necessarily make them safer. According to the U.S. DOE Sandia National Laboratories, solid electrolytes can still fail under certain conditions. This happens when the battery is crushed, punctured or when the internal pressure builds up to a level that makes the lithium react with the internal oxygen.
Regardless of whether they have a solid or liquid electrolyte, all Li-Ion batteries form dendrite spikes under normal use. They have the bad habit of puncturing through the layers of separator in the battery, causing a short circuit. When the solid electrolyte does fail, the resulting short circuit will lead to the same heat build-up as in the case of a conventional Li-Ion battery.
In some cases, short-circuited solid-state batteries can reach temperatures significantly higher than conventional Li-ion, shows the Sandia study published in the scientific journal Joule. This happens because there’s a huge amount of energy stored in the battery that gets released when the positive and the negative poles are no longer separated by the electrolyte. There’s no way around that, and although the electrolyte is nonflammable, other surrounding materials might be.