Despite controversies, hydrogen can be a great fossil fuel replacement and also a means of energy storage and transport. But extracting hydrogen is an energy-intensive process and most of the time not entirely environmentally friendly. A breakthrough in concentrated solar power could provide the solution to produce green hydrogen via a new thermochemical process.
Despite being the most abundant element on Earth, hydrogen is not easy to get, because it only exists in combination with other materials. Right now, hydrocarbons and water are the preferred substances to obtain hydrogen, and both processes have critical flows. Cracking hydrocarbons to get hydrogen is the cheapest way, but besides hydrogen, carbon is also generated. When this carbon is captured, we have “blue hydrogen” but most hydrogen is grey, which means the carbon is released into the atmosphere.
Of course, there’s also the green hydrogen, which is generated by water electrolysis using renewable energy sources. Unfortunately, breaking the water molecules requires huge amounts of energy. It’s a very expensive process and this explains why it only accounts for less than 5% of the hydrogen used today. Fortunately, scientists are hard at work with a new way of getting green hydrogen, called solar thermochemical hydrogen (STCH) production.
The new method proposed by the scientists at the Department of Energy’s National Renewable Energy Laboratory (NREL) is potentially more energy-efficient than producing hydrogen via the commonly used electrolysis method. This could allow cutting the cost of clean hydrogen by 80% to $1 per kilogram in the next decade, a goal outlined by the Department of Energy’s Hydrogen Energy Earthshot Initiative.
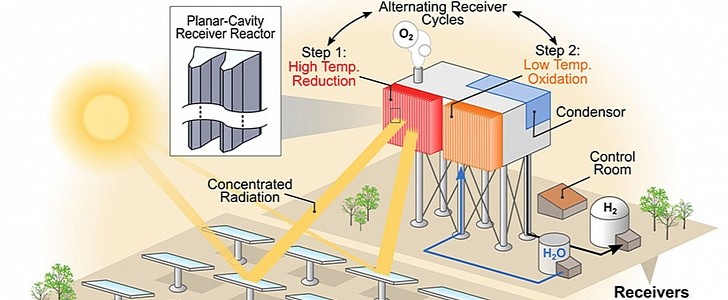
The electrolysis uses electricity to split water into hydrogen and oxygen but STCH relies on a two-step chemical process in which metal oxides are exposed to temperatures greater than 1,400 degrees Celsius and then re-oxidized with steam at lower temperatures to produce hydrogen. Getting to such a high temperature might seem problematic and just as energy-consuming as the electrolysis, but fortunately, this is not the case.
Instead of using solar energy to produce electricity, the STCH relies on concentrated solar power to use heat directly. More specifically, a field of specialized mirrors reflects the sun rays and concentrates them on a smaller point, generating heat way beyond the capabilities of typical fossil power plants.
Unlike the photovoltaic panels (PV), which use only a limited section of the solar spectrum, STCH can use the entire spectrum. This leads to significantly higher efficiency. But before you get too excited, we must stress that the STCH process is still in its early stages of development. The main obstacle at the moment is finding the right materials that can provide high performance under high heat.
“It’s certainly a very challenging field, and it has a lot of research questions still unanswered, mainly on the materials perspective,” said Zhiwen Ma, a senior engineer at NREL and lead author of a new paper, “System and technoeconomic analysis of solar thermochemical hydrogen production,” which appears in the journal Renewable Energy.
According to the NREL, perovskite materials may hold the potential to play an important role in efficient STCH production. Perovskites refer to a class of lab-grown crystalline materials with interesting and intriguing properties. NREL research deep-dives into the perovskite field to identify the best materials in terms of performance and costs.
Despite Elon Musk’s beliefs, NREL thinks that hydrogen could be an important carrier to store energy generated by renewable resources, as a substitute for fossil fuels used for transportation, in the production of ammonia, and for other industrial applications. The only missing link is how to get it in a cost-efficient manner and with zero carbon impact on the environment.
Of course, there’s also the green hydrogen, which is generated by water electrolysis using renewable energy sources. Unfortunately, breaking the water molecules requires huge amounts of energy. It’s a very expensive process and this explains why it only accounts for less than 5% of the hydrogen used today. Fortunately, scientists are hard at work with a new way of getting green hydrogen, called solar thermochemical hydrogen (STCH) production.
The new method proposed by the scientists at the Department of Energy’s National Renewable Energy Laboratory (NREL) is potentially more energy-efficient than producing hydrogen via the commonly used electrolysis method. This could allow cutting the cost of clean hydrogen by 80% to $1 per kilogram in the next decade, a goal outlined by the Department of Energy’s Hydrogen Energy Earthshot Initiative.
The electrolysis uses electricity to split water into hydrogen and oxygen but STCH relies on a two-step chemical process in which metal oxides are exposed to temperatures greater than 1,400 degrees Celsius and then re-oxidized with steam at lower temperatures to produce hydrogen. Getting to such a high temperature might seem problematic and just as energy-consuming as the electrolysis, but fortunately, this is not the case.
Instead of using solar energy to produce electricity, the STCH relies on concentrated solar power to use heat directly. More specifically, a field of specialized mirrors reflects the sun rays and concentrates them on a smaller point, generating heat way beyond the capabilities of typical fossil power plants.
Unlike the photovoltaic panels (PV), which use only a limited section of the solar spectrum, STCH can use the entire spectrum. This leads to significantly higher efficiency. But before you get too excited, we must stress that the STCH process is still in its early stages of development. The main obstacle at the moment is finding the right materials that can provide high performance under high heat.
“It’s certainly a very challenging field, and it has a lot of research questions still unanswered, mainly on the materials perspective,” said Zhiwen Ma, a senior engineer at NREL and lead author of a new paper, “System and technoeconomic analysis of solar thermochemical hydrogen production,” which appears in the journal Renewable Energy.
According to the NREL, perovskite materials may hold the potential to play an important role in efficient STCH production. Perovskites refer to a class of lab-grown crystalline materials with interesting and intriguing properties. NREL research deep-dives into the perovskite field to identify the best materials in terms of performance and costs.
Despite Elon Musk’s beliefs, NREL thinks that hydrogen could be an important carrier to store energy generated by renewable resources, as a substitute for fossil fuels used for transportation, in the production of ammonia, and for other industrial applications. The only missing link is how to get it in a cost-efficient manner and with zero carbon impact on the environment.