The times we live in are simply astounding. A recent press release from Audi claims that the Ingolstadt-based company managed to obtain a form of diesel out of CO2. If the process they describe is as viable and easy as they claim, this will solve a number of problems on a worldwide level.
Just last year, the UN ranked global warming amongst the top risk factors for the humanity itself. This is the first time when political factors agree on the fact that pollution and CO2 emissions are killing us off, one small iceberg at a time. Imagine factories that would be able to take that CO2 and use it to create fuel for our cars.
That would not only help out with the pollution issues, but it would also solve the problem of ever-diminishing fossil fuel reserves that has the whole world all scared at the moment. Audi claims they have actually succeeded in doing so.
To celebrate this achievement, Germany’s own Federal Minister of Education and Research Prof. Dr. Johanna Wanka took it upon herself to use the first five liters of e-diesel in her car, an Audi A8 TDI.
“This synthetic diesel, made using CO2, is a huge success for our sustainability research. If we can make widespread use of CO2 as a raw material, we will make a crucial contribution to climate protection and the efficient use of resources, and put the fundamentals of the “green economy” in place,” declared Wanka.
The plant created by Audi in Dresden managed to obtain the aforementioned synthetic diesel after just four months, claiming that the process was fueled by regenerable energy alone.
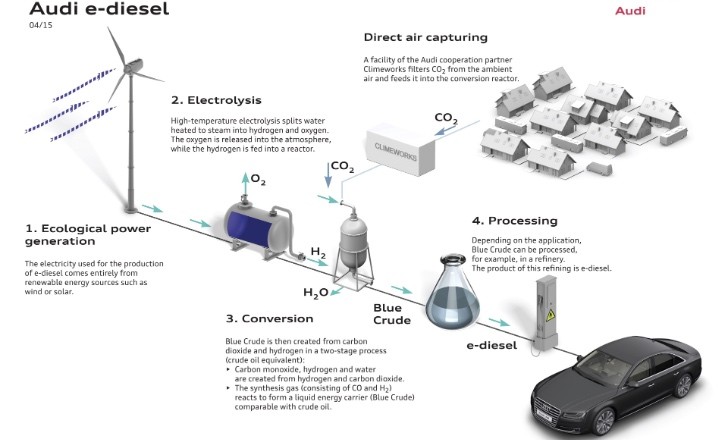
Using the Power to Liquid principle, the engineers and chemists claim that only water and carbon dioxide are needed to create e-diesel. Unfortunately, at the moment, only a part of the CO2 used comes from direct air capturing, the rest being provided by a biogas facility.
So how is this done? Well, first water is heated up to form steam. This in turn is then broken down into hydrogen and oxygen by means of high-temperature electrolysis. This process, involving a temperature in excess of 800 degrees Celsius, is more efficient than conventional techniques because of heat recovery, for example. Another special feature of high-temperature electrolysis is that it can be used dynamically, to stabilize the grid when production of green power peaks.
From here the hydrogen reacts with the CO2 in synthesis reactors in certain conditions (under pressure and at high temperatures). The result is a liquid made from long-chain hydrocarbon compounds that’s called blue crude.
Due to its characteristics, blue crude can be used just like a fossil crude oil and it is refined to obtain the e-diesel final product. What makes this kind of process better than, say biodiesel, is its high efficiency at around 70 percent.
That would not only help out with the pollution issues, but it would also solve the problem of ever-diminishing fossil fuel reserves that has the whole world all scared at the moment. Audi claims they have actually succeeded in doing so.
To celebrate this achievement, Germany’s own Federal Minister of Education and Research Prof. Dr. Johanna Wanka took it upon herself to use the first five liters of e-diesel in her car, an Audi A8 TDI.
“This synthetic diesel, made using CO2, is a huge success for our sustainability research. If we can make widespread use of CO2 as a raw material, we will make a crucial contribution to climate protection and the efficient use of resources, and put the fundamentals of the “green economy” in place,” declared Wanka.
The plant created by Audi in Dresden managed to obtain the aforementioned synthetic diesel after just four months, claiming that the process was fueled by regenerable energy alone.
Using the Power to Liquid principle, the engineers and chemists claim that only water and carbon dioxide are needed to create e-diesel. Unfortunately, at the moment, only a part of the CO2 used comes from direct air capturing, the rest being provided by a biogas facility.
So how is this done? Well, first water is heated up to form steam. This in turn is then broken down into hydrogen and oxygen by means of high-temperature electrolysis. This process, involving a temperature in excess of 800 degrees Celsius, is more efficient than conventional techniques because of heat recovery, for example. Another special feature of high-temperature electrolysis is that it can be used dynamically, to stabilize the grid when production of green power peaks.
From here the hydrogen reacts with the CO2 in synthesis reactors in certain conditions (under pressure and at high temperatures). The result is a liquid made from long-chain hydrocarbon compounds that’s called blue crude.
Due to its characteristics, blue crude can be used just like a fossil crude oil and it is refined to obtain the e-diesel final product. What makes this kind of process better than, say biodiesel, is its high efficiency at around 70 percent.